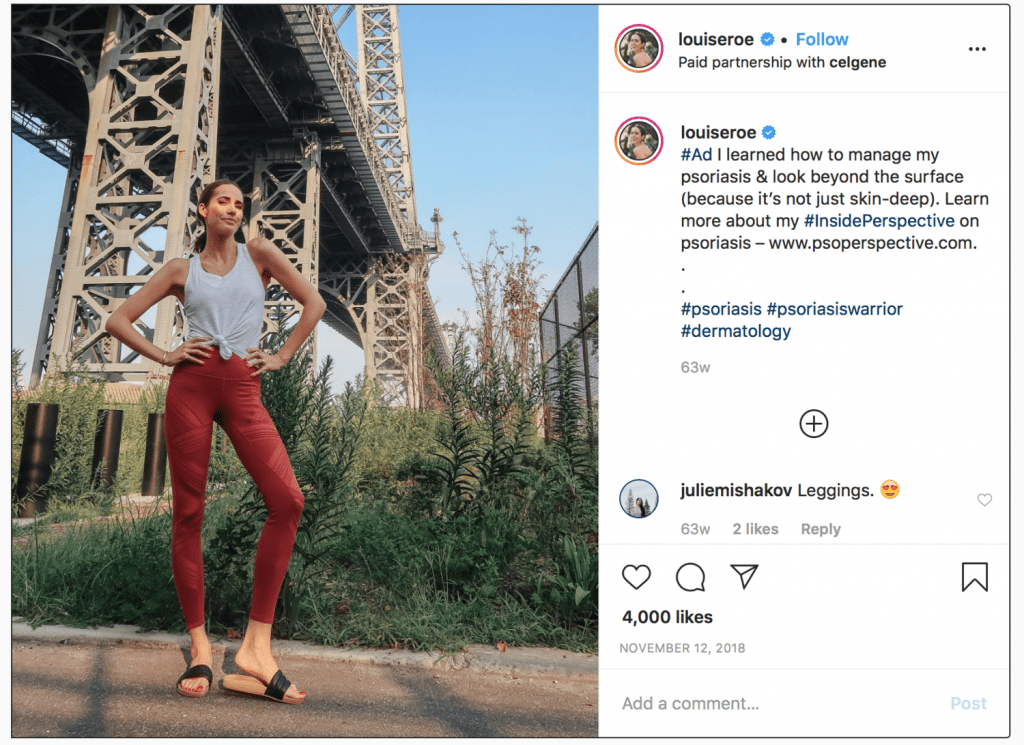
Forget Bottega Veneta handbags and the trend-specific wares from retailers like Revolve for a moment. A growing number of influencers have taken to hawking something else entirely: pharmaceuticals. Whether it be mega-famous stars promoting morning sickness medication or highly-followed figures talking up medical remedies for psoriasis or endometriosis, big pharma is readily looking beyond its traditional advertising avenues to the burgeoning influencer market – which is estimated to reach $22.3 billion in value by 2024 – to sell more drugs.
And selling more drugs they are. According to health-oriented news site Stat, when Kim Kardashian posted about morning sickness medication Diclegis on her Instagram in 2015 (in exchange for a reported $500,000 payment), drug-maker Duchesnay was able to rack up nearly half a million likes and boost the social media conversation about its drug by 500 percent by way of Kardashian’s endorsement. More than that, Stat says that sales for the prescription medication jumped 21 percent to nearly $41.7 million.
The ad campaign – which ultimately saw the Food and Drug Administration (“FDA”) send a warning letter to Diclegis-maker Duchesnay because Kardashian failed to properly disclose any of the legally-required risk information or important limitations of use for the drug – was “a high-profile example of how influencers can hold sway over the public at large,” per Stat.
Looking beyond the Kardashian effect, just how effective is influencer advertising when it comes to pharmaceuticals? That is precisely what the FDA wants to know – and plans to find out. The government agency – which is responsible for “protecting the public health by assuring the safety, efficacy and security of drugs, biological products, medical devices, and the nation’s food supply,” among other things, and thereby, has regulatory power over the promotion of pharmaceuticals by celebs and influencers – has revealed that it is set to launch a study aimed at dissecting the connection between paid pharmaceutical endorsements and consumer behavior.
As part of a formal study, the FDA revealed in a release on Tuesday that it is planning to examine the promotion of medications by celebrities, physicians, patients and online influencers to determine the extent to which paid endorsements affect consumers, including their “attention to disclosure statements and risk/benefit information; retention of risk/benefit information; recognition of [the nature of the content as a] promotion and endorser as [having been] paid; perceived benefits and risks, attitudes toward the product, endorser, and ad; and behavioral intentions, such as asking a doctor about the drug.”
One aspect of the agency’s two-part study, which is slated to commence this fall, according to a spokesman for the FDA, will compare consumer responses to pharma-related posts from different types of endorsers. This will be tested by showing survey participants “an Instagram post for a fictitious endometriosis product,” and manipulating an array of factors, including “the explicitness of the payment disclosure,” and the endorsers, themselves, including “celebrities, physicians, patients and online influencers.”
Meanwhile, the evolution of pharmaceutical advertising to include social media-centric campaigns paired with the fact that influencers are not only willing and able to impact consumers purchasing decisions when it comes to designer footwear and exotic vacation destinations, but to actually influence their medical decisions has other regulators on high alert, as well.
Advertising experts expect that the Federal Trade Commission, a separate federal entity, will make this an area of increased oversight. While the government agency – which is tasked with promoting consumer protection, and eliminating and preventing anticompetitive business practices – has been relatively lax when it comes to the state of influencer disclosures (and the widespread lack thereof), given the high stakes that come with the promotion of medication, this is likely one place where we can expect to see increased attention in the not too distant future.